Hero
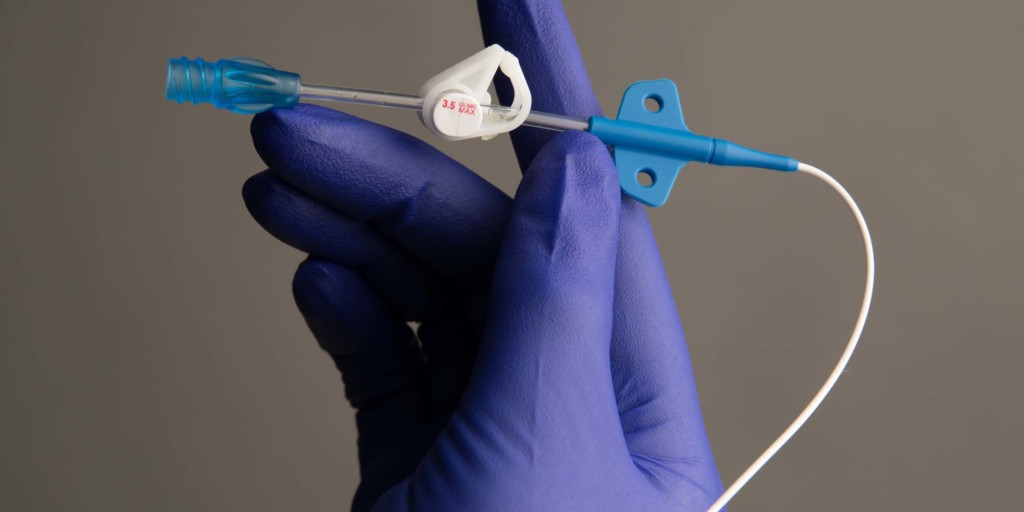
Access Vascular has announced that it has received clearance from the FDA for the second generation of its HydroPICC peripherally inserted central catheter (PICC), which has demonstrated a thrombus accumulation rate 30 times less than traditional PICCs and the potential to significantly reduce catheter occlusions.
PICCs have become an increasingly popular choice over traditional central venous lines due to ease of use, decreased risk, reduced cost and ability to be placed by non-physicians. However, traditional PICCs are more than twice as likely to experience catheter-related thrombus, leading to significant patient complications and associated costs. They are comprised of polyurethanes or silicones that absorb proteins, which attract thrombus. The inherent characteristics of these materials may also cause phlebitis and catheter-related infections, as well as other PICC line complications.
HydroPICC is comprised of Access Vascular’s patented biomaterial platform, which combines the superior mechanical properties of polyurethanes with the intrinsically low thrombogenicity of hydrogel. The lubricious and hydrophilic material is designed to repel protein and thrombus development, prevent catheter occlusion, reduce infections and add flexibility to the catheter.
According to a statement, the company plans to develop a variety of thrombus-resistant venous catheters and devices from the biomaterial to address the US$4.4 billion venous access market, including midlines, peripherally inserted venous catheters, ports, central venous catheters and dialysis catheters.
The biomaterial also has the future potential to incorporate sustained delivery of such therapeutics as anti-infectives, analgesics/anaesthetics or anti-inflammatories.
“The importance of improving the biocompatibility of materials that reside within the intravascular space cannot be overemphasized,” said Gregory J Schears, a professor of anaesthesiology in Rochester, USA and medical advisor to Access Vascular.
“Despite attention to optimal site choice and catheter-to-vein ratios, patients often develop fibrin sheaths that inhibit catheter functionality. They also commonly develop venous thrombus, sometimes with associated bloodstream infections, that use these depositions as basecamps for biofilm formation.”
A material that could combat these issues, Schears revealed, has the potential to “significantly improve vascular access throughout the body”.
The second-generation device is easy to insert and integrates new features that streamline placement time based on clinician feedback gleaned from the commercial experience of the first-generation catheter.
The company will focus on generating clinical evidence at a limited number of commercial sites in the USA in 2020. “We are excited about the potential of our biomaterial to address significant and under-served clinical needs in venous access,” said Access Vascular CEO James Biggins.





