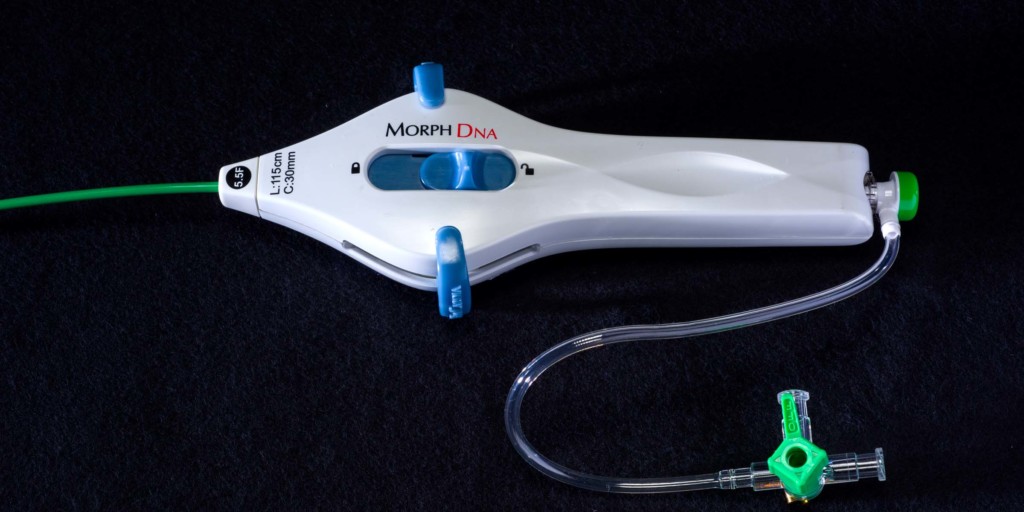
Morph DNA
BioCardia has announced that the FDA has granted 510(k) clearance for the Morph DNA deflectable guide catheter, which is used to guide the Helix biotherapeutic delivery system during CardiAMP cell therapy delivery in the heart.
The Morph DNA deflectable guide catheter, a press release reports, is designed to enhance procedural control of therapeutic catheters, such as the Helix system, during delivery of cell therapy. The press release adds that it is intended to provide superior ease of use through bidirectional deflection, reduced torque build up or whip, enhanced fluoroscopic visibility, and improved ergonomics. Helically-arranged pull-wires in the device, resembling the double helix of a DNA strand, increase catheter stability, rendering it virtually whipless around curves and within the heart. The device also features a new handle design with an adjustable brake for finer catheter control during procedures.
Gerald Koenig (Henry Ford Health System, USA), principal investigator of the CardiAMP Heart Failure Trial, says: “The Morph DNA deflectable guide enables navigation within the cardiac chambers without building up torque. This is hugely beneficial for controlling the delivery of therapeutic catheters, such as Helix. The ergonomic features simplify the handling and can reduce procedural delays.”
BioCardia CEO Peter Altman, notes: “Our investigational Helix delivery system using the original Morph technology has shown a great safety profile and has been shown to be dramatically more efficient in helping the heart retain cell therapy when compared to other leading delivery approaches. The new Morph DNA device is an elegant product intended to further enhance the performance of our biotherapeutic delivery capabilities, and through these, our CardiAMP and CardiALLO cell therapy programmes.”





