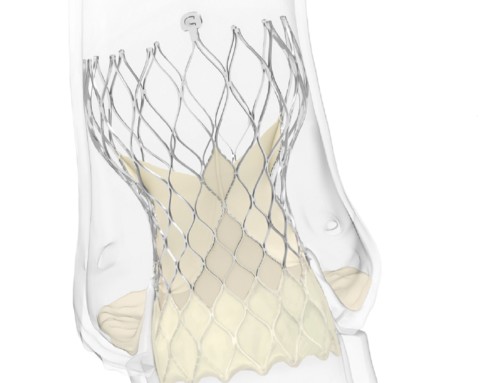
Cordella system
Endotronix has announced the expansion of its Series D financing round, bringing the total for the round to $70 million. The expansion syndicate for the LSP-led round includes new investment from an additional unnamed medical device strategic investor as well as participation from existing investors, including Aperture Venture Partners, BioVentures Investors, Lumira Ventures, OSF Ventures, Seroba Life Sciences, Skydeck LLC, SV Health Investors, Wanxiang Healthcare Investments, and an unnamed corporate strategic investor.
A press release reports that the funds will be used to support the landmark PROACTIVE-HF IDE clinical trial of the Cordella pulmonary artery sensor system (Cordella sensor) and commercialisation of the Cordella heart failure system (Cordella system).
It states that the Cordella system is designed to help patients with heart failure feel better and stay out of the hospital with streamlined care and remote medication titration. The system is designed to provide a comprehensive health status of the patient at home with easy-to-use tools to securely collect and share health-related data with healthcare providers for trend-based management. The Cordella sensor integrates pulmonary artery pressure data into the Cordella system. Together, they aim to proactively deliver the information necessary to improve patient care between office visits and support reimbursement for care delivery activities.
Fouad Azzam, general partner at LSP, says: “Endotronix has built a strong syndicate of both institutional and strategic investors who believe in the benefits of remote heart failure management with the Cordella sensor and system. The team is set to deliver an elegant solution that we believe will show a definitive benefit in reducing heart failure related hospitalisations and improving outcomes for patients and clinicians.”
Endotronix has two active clinical studies for the Cordella sensor:
- PROACTIVE-HF is a prospective, multicentre, randomised, controlled, single-blind trial that will evaluate the safety and efficacy of the Cordella sensor in more than 950 New York Heart Association (NYHA) Class III heart failure patients at up to 60 sites across the USA. The trial has received FDA approval and enrolment is expected to begin in the fourth quarter of 2019. The pivotal trial is designed to show a definitive benefit for pulmonary artery pressure-guided management in the reduction of heart failure hospitalisations and mortality as well as provide the data required for access to the US market and reimbursement of the implantable Cordella sensor across the USA.
- The open-label SIRONA II CE mark trial is currently enrolling 60 patients at up to eight European medical centres. The trial will evaluate the safety and efficacy of the Cordella Sensor for the management of NYHA class III heart failure patients and provide data for market access to the European market.
The Cordella system, without the sensor, is available for commercial use in the USA and Europe and is currently in cardiology centres across the USA. The Cordella sensor is an investigational device and is not available for commercial use in any geography.